|
Seroprevalence of Brucella canis and Leptospira spp. in canines in the city of Medellín, Colombia*
Laura López-Diez1
1 Biogenesis Research Group. Department of Agrarian Sciences, Universidad de Antioquia. Medellín, Colombia 2 Department of Agrarian Sciences, Universidad de Antioquia. Medellín, Colombia 3 Technical Area for the Prevention and Control of Zoonoses, Medellín, Colombia
This email address is being protected from spambots. You need JavaScript enabled to view it..
Recibido: 9 septiembre de 2019, Aprobado: 30 noviembre de 2019, actualizado: 17 diciembre de 2019
DOI: 10.17151/vetzo.2020.14.1.4
ABSTRACT. Introduction: The agents that cause diseases of zoonotic importance in canines, such as Canine brucellosis and Leptospirosis, have gained importance in human clinical practice. Objective: To discover the prevalence and behavior of both diseases in the canine population in the city of Medellín to develop measures of prevention and control in this area. Methods: A total of 1,300 canines were sampled to test for Brucella canis and Leptospira spp. using the PARP-2ME and MAT techniques, respectively, to establish the statistical significance of the different variables analyzed (P ≤ 0.05; OR ≥ 1; 95% CI). Results: Seroprevalence was determined to be 7.32% for B. canis and 9.08%, for Leptospira spp. with a 0.77% co-infection rate of both diseases. The most prevalent serovars for Leptospira spp. were Canicola (3.38%), Icterohaemorrhagiae (2.62%), and Pomona (0.92%). A statistical association was reported for B. canis with the commune variable (San Javier P = 0.002; OR = 2.724 / Guayabal P = 0.000; OR = 3.862 / Belén P = 0.002; OR = 2.953), and for Leptospira spp. with the commune variable (Buenos Aires P = 0.011; OR = 2.220) and age (37-48 months P = 0.005; OR = 4.272). Conclusions: This study shows that both agents are in circulation among the canine population in the city and in all the communes analyzed, representing a possible risk of infection to owners and other animals entering into contact with them.
Key words: brucellosis; leptospirosis; dogs; prevalence; zoonosis.
Seroprevalencia de Brucella canis y Leptospira spp. en caninos de la ciudad de Medellín, Colombia
RESUMEN. Introducción: los agentes causantes de enfermedades de importancia zoonótica en caninos como la Brucelosis canina y la Leptospirosis han cobrado importancia en la clínica humana. Objetivo: conocer la prevalencia y comportamiento de ambas enfermedades en la población canina de la ciudad de Medellín, para promover el desarrollo de medidas preventivas y de control en esta. Métodos: 1300 caninos fueron muestreados para el análisis de Brucella canis y Leptospira spp. por medio de las técnicas PARP-2ME y MAT, respectivamente; siendo establecida la significancia estadística con las diferentes variables analizadas (P≤0,05; OR≥1; IC 95%). Resultados: se evidenció una seroprevalencia para B. canis del 7,32% y para Leptospira spp. del 9,08%, con una coinfección entre ambas enfermedades del 0,77%. Los serovares más prevalentes para Leptospira spp. fueron Canicola (3,38%), Icterohaemorrhagiae (2,62%) y Pomona (0,92%). Se halló asociación estadística para B. canis con la variable comuna (San Javier P=0,002; OR=2,724 / Guayabal P=0,000; OR=3,862 / Belén P=0,002; OR=2,953); para Leptospira spp. con la variable comuna (Buenos Aires P=0,011; OR=2,220) y edad (37-48 meses P=0,005; OR=4,272). Conclusiones: el estudio demuestra la circulación de ambos agentes en la población canina de la ciudad y en todas las comunas analizadas, lo cual representaría un posible riesgo de infección para los propietarios y otros animales que entren en contacto con estos.
Palabras claves: brucelosis, leptospirosis, perros, prevalencia, zoonosis.
Introduction
Currently, Medellín has no regulations that prevent or control brucellosis and leptospirosis among companion animals (Echeverri et al., 2017; Olivera & Di-Lorenzo, 2009). This problem indicates that stray dogs, semi-domestic dogs, kennels, and canine shelters with no regulations, continue to be a risk factor for infection for the humans and animals that come into contact with positive individuals (Castrillón et al., 2013; Ruíz et al., 2010). Note that both diseases have been reported in humans and canines in the city, indicating that investigating the behavior of these diseases is essential if an approach to preventive measures is to be achieved.
Canine brucellosis is the most important reproductive disease in dogs, caused by a gram-negative intracellular bacterium called Brucella canis. It can be asymptomatic in humans or it can cause nonspecific symptoms including fever, fatigue, arthritis, lymphadenopathy, malaise, cough, myalgia, eye lesions, anemia, orchitis, epididymitis, nephritis, and neurological symptoms (Sánchez et al., 2013; Souza et al., 2018). A 5% mortality rate has been reported in humans from serious conditions such as endocarditis and meningitis (Lucero et al., 2010; Manias et al., 2013). In canines, the disease may manifest as miscarriage, orchitis, epididymitis or prostatitis, infertility, small or weak litters, or litters that die soon after birth, arthritis, discoespondylitis, osteomyelitis, fever, lymphadenopathy, and eye decay and infection (Ardoino et al., 2017; Souza et al., 2018). The most vulnerable human population includes veterinarians, veterinary laboratory personnel, kennels, and pet owners who are positive. The disease can spread to them through contact with vaginal, preputial, seminal, placental, or fetal secretions during delivery or abortion, or the saliva, urine, feces, and milk of infected animals. Canines can become infected with these same secretions through the genital, oronasal, and conjunctival mucosa (Giraldo et al., 2009). Puppies from positive mothers also represent a source of dissemination of the bacterium (Souza et al., 2018). Among canines, a prevalence in Latin America of between 3.3% and 30.5% has been evidenced in countries such as Argentina, Brazil, Peru, and Mexico (Agudelo et al., 2012; Castrillón et al., 2013). In Colombia, it ranges from 1.4 to 20.3% among the pet population, kennels, and shelters. In Medellín, seroprevalences of 8.9%–15% in canines have been reported in kennels, 11% in samples sent to veterinary laboratories, 15% in samples from veterinary clinics, 6.8% in shelters and, for the Aburrá Valley, from 17.2% to 27.7% overall (Castrillón et al., 2013; Olivera et al., 2011; Sánchez et al., 2013).
Leptospirosis is attributed to a spirochete of the species Leptospira interrogans comprising pathogenic serovars that develop severe clinical symptoms in 10%-20% of humans, with a reported mortality in Colombia of between 1.5% and 5% (Echeverri et al., 2017). In humans, the symptoms are non-specific such as fever, diarrhea, headaches, jaundice, myalgia, meningitis, lymphadenopathy, vomiting, hepatosplenomegaly, kidney and liver failure, pulmonary involvement, and Weil’s syndrome. In canines, the disease manifests itself with anorexia, polydipsia, vomiting, myalgia, fever, kidney failure, jaundice, bleeding, ulcers, halitosis, abdominal pain, and respiratory disorders (Álvarez et al., 2011; Echeverri et al., 2017). Symptoms can vary as per the serovar that affects the host. It has also been considered an occupational disease for the agricultural sector and is related to climate and environmental change, poor living conditions, and urban expansion (Carreño et al., 2017; Miotto et al., 2018). Animals and humans can be infected by contact with water, ground, soil, or food that has been contaminated with urine, blood, and infected tissues, through oral, nasal, conjunctival, and genital mucosa, or skin lesions. At a global level, an incidence of 0.1-1 per 100,000 inhabitants is stipulated in temperate climates and 10 for every 100,000 inhabitants in tropical climates. In Colombia, there is evidence of underreporting in the Public Health Surveillance System (Sivigila), which interferes with the actual estimate of the prevalence of this disease in Colombia. A prevalence of 6%–67.9% in humans has been identified and 12%–67.2% in canines, with the most reported serovares being Icterohaemorrhagiae, Grippotyphosa, and Canicola (Carreño et al., 2017; Echeverri et al., 2017; Pulido et al., 2014). In the Department of Antioquia, a prevalence of between 12.5% and 62.1% has been reported in humans, making it one of the highest in the country (Carreño et al., 2017; Pulido et al., 2014).
No seroprevalences have been identified for canines in the department or city under study. Both diseases show that underdiagnosis, nonspecific symptoms, direct and indirect transmission, and inefficient therapy enables healthy carriers to constantly spread the agent and represent a risk to owners and animals (Ardoino et al., 2017; Echeverri et al., 2017). The aim of this study was to discover the seroprevalence of both diseases to help begin an epidemiological surveillance program that would enable strategies to be developed to mitigate the risk of human–animal companion transmission.
Materials and Methods
Population and area of study
A cross-sectional study was performed, with a sample of 1,300 apparently healthy canines that were taken to the Sterilization Conference at Medellín’s City Hall from 2016 to 2018. The number of animals was distributed among fifteen of the sixteen communes that make up Medellín; commune 12 (La América) and the surrounding townships were not included in the sample. The number of samples and the communes to be analyzed were determined by convenience; 100 samples were collected from communes 1, 2, 3, 8, and 13; however, in the others, 80 samples were taken per commune.
Sample collection
Whole blood samples were collected in MiniCollet® tubes with a red lid after canalizing the cephalic vein during sterilization. The samples were later taken to the laboratory and centrifuged at 1,361 g for 5 min to separate the serum, which was stored in 1.5 ml Eppendorf tubes at -20°C until due processing.
Diagnosis
The Rapid Plate Agglutination Technique was applied to all samples with 2 β -Mercaptoethanol (PARP-2ME), using the Microagglutination (MAT) Technique, for the diagnosis of Brucella canis and Leptospira interrogans spp., respectively. A description is provided in the study by Castrillón et al, 2019 (Castrillón et al., 2019) The serovars of Leptospira spp. included in the analysis were Canicola, Icterohaemorrhagiae, Grippotyphosa, Pomona, Ballum, Autumnalis, Bratislava, and Tarassovi. Positive individuals were those who showed an agglutination similar to that of the positive control in the B. canis test; for Leptospira spp.; it included individuals who obtained titers ≥1: 100. In commune 3, three samples for B. canis diagnosis were discarded because of hemolysis, leaving in total only 97 samples for this commune.
Statistical analysis
SPSS® version 25 was used for the analysis. Initially, a descriptive analysis was performed to identify the number of dogs that tested positive for Brucella canis or Leptospira spp. according to commune, stratum, age, and gender. Subsequently, a binary logistic regression analysis was performed using a 95% confidence interval to determine which ones behaved as a risk factor or a protective factor among independent variables (gender, age, commune, and stratum) and dependent variables (positive or negative). To facilitate the analysis, ages were grouped into the ranges of 1-6 months, 7-12 months, 13-24 months, 25-36 months, 37-48 months, and ≥49 months.
Results and Discussion
A total of 580 females and 720 males were sampled, of which 62.45% were within an age range of 7-24 months. A seroprevalence of 7.32% was evidenced for B. canis and of 9.08% for Leptospira spp., with 0.77% coinfection of both diseases. The seroprevalence reported for B. canis (7,32%) was similar to what was previously reported in the city, in Colombia, and in countries such as Argentina (7.3%–30.5%) and Peru (3.3%–28%). However, it is below what was reported for Mexico (11%–28%) and Brazil (14.2%) (Agudelo et al., 2012; Castrillón et al., 2013; Castrillón et al., 2019). Moreover, for Leptospira spp. (9.08%), the seroprevalence reported was lower than what was reported in previous studies for Colombia and other countries such as Brazil and Nicaragua (Langoni et al., 2015; Miotto et al., 2018). However, the values remain similar to a preliminary study performed in five communes in Medellín (Carreño et al., 2017; Castrillón et al., 2019). The low availability of recent studies of this nature in Colombia and in other countries makes it complex to actually determine the degree of divergence of seroprevalence in other canine populations throughout the world. Furthermore, variations in prevalence may be attributed to the canine population studied or to the Diagnostic Techniques used in the other studies.
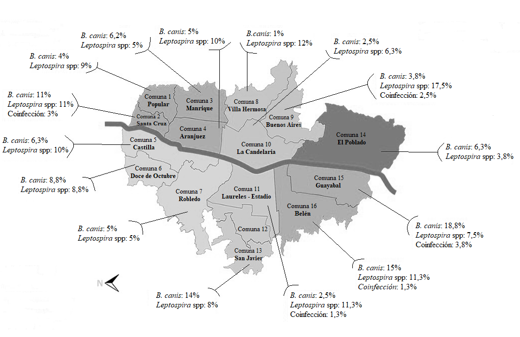
Figure 1 shows the distribution of seroprevalence for both agents by commune. The communes with the highest seroprevalence for B. canis and Leptospira spp., corresponded to Guayabal and Buenos Aires, respectively. The canines who were reported to be seropositive to both agents in all sampled communes reflects that there is a permanent circulation of these agents among the population, which could represent a possible source of infection in susceptible humans and animals within the city. Previously, the ownership of canines infected by B. canis promoted the transmission of the infectious agent to its owners (Giraldo et al., 2009; Olivera & Di-Lorenzo, 2009). Furthermore, the latest INS epidemiological report indicated that 50.5% of individuals positive for leptospirosis had prior contact with dogs and 59.3% with rodents (Salas, 2018); Similarly, dog ownership has been identified as a risk factor for Leptospira spp. in humans in countries such as Germany, Barbados, and Nicaragua (Delaude et al., 2017). Although it is not possible to precisely determine that contact with this species is one of the main sources of infection for brucellosis and leptospirosis in humans, it is recognized that the capacity of these agents to generate subclinical conditions with consequent intermittent bacterial shedding would be a possible risk factor associated with the presentation of human cases (Flores et al., 2017; Miotto et al., 2018; Souza et al., 2018).
Haga clic sobre la imagen para ampliarla Figure 1. Seroprevalence for B. canis and Leptospira interrogans spp. by commune among canines in the city of Medellín.
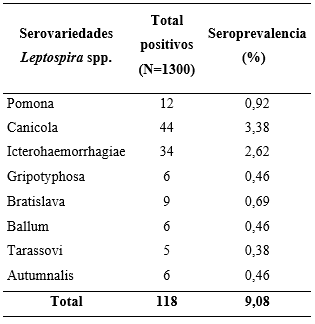
As shown in Table 1, positivity was evidenced in all the serovars of Leptospira interrogans spp. analyzed with the most prevalent being Canicola (3.38%), Icterohaemorrhagiae (2.62%), and Pomona (0.92%). Four of the individuals studied exhibited seropositivity to more than one serovar and reported the following combinations: Canicola-Icterohaemorrhagiae (Commune 9); Canicola-Ballum (Commune 6); Ballum-Pomona (Commune 1); Grippotyphosa-Bratislava (Commune 2). The above reflects that canines are possible carriers and disseminators of different serovars of Leptospira spp., which can seriously affect human beings.
Antibody detection through MAT is not related to spirochete clearance in urine either because of a low immune response associated with its location or because of the possible interference from vaccine antibodies. However, it has been determined that it is possible to identify canines that, despite having low or null antibody titers, present bacterial elimination that promotes the contamination of the environment and a contagion risk for susceptible humans and animals (Flores et al., 2017; Miotto et al., 2018). In this study, it was not possible to exclude possible post-vaccine antibody interference because of this information not being included; however, some researchers report that there is low interference from vaccination because the titers generated by them are lower than those detected with this technique or because they decrease rapidly over a period of 3-6 months (Miotto et al., 2018).
Table 1. Seroprevalence against the serovars of Leptospira interrogans spp. *0.29% of the individuals were positive to more than one serovar.
The most prevalent serovars in canines (L. canicola and L. icterohaemorrhagiae) coincided with what was previously reported by other authors, with Icterohaemorrhagiae being considered as the most pathogenic for humans, because it has been associated with greater renal and hepatic involvement. This does not rule out the clinical significance of L. canicola which includes previous reports of disease in humans (Álvarez et al., 2011; Carreño et al., 2017; Flores et al., 2017; Langoni et al., 2015). Both serovars have been considered to be the most prevalent in humans (Carreño et al., 2017; Castrillón et al., 2019). As rodents are reservoirs of serovar L. icterohaemorrhagiae, it could be supposed that there is a direct or indirect transmission from them to canines with the latter acting as disseminators among the different populations (Castrillón et al., 2019). The serovar L. pomona, the third most prevalent in this study, has been reported as having the highest record in humans in the center of the department of Antioquia, and L. grippotyphosa has been reported mostly in its coastal region (Echeverri et al., 2017; Flores et al., 2017).
In a study performed in the department of Tolima, L. Pomona is the most prevalent serovar in humans and the second most prevalent in canines (Carreño et al., 2017). The serovars L. pomona and L. grippotyphosa are specific to pigs and cattle, which makes the presence of antibodies in the canines studied questionable. As they are in an urban location, there would be no direct contact with the mentioned species, which may represent possible anthropozoonosis for the canines. Because they are susceptible to all Leptospira spp. serovars, they may behave as carriers and disseminators (Álvarez et al., 2011; Castrillón et al., 2019; Flores et al., 2017).
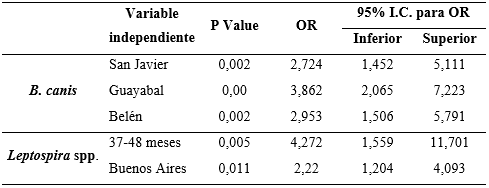
The logistic regression analysis failed to show a statistical association between gender or socioeconomic stratum with positivity to any of the agents studied. However, the “commune” variable was reported to be a risk factor, indicating that canines from the San Javier, Belén, and Guayabal communes are between 2.7 and 3.8 times more at risk of being infected with B. canis in comparison with dogs from the other communes studied. Similarly, canines in the Buenos Aires commune have 2.2 times higher risk of infection with Leptospira spp. than those who live in the other communes (Table 2).
Table 2. Association between the dependent and independent variables for Brucella canis and Leptospira spp.
In the study carried out by Agudelo et al. (2012), a higher prevalence was evidenced for B. canis in the communes of Buenos Aires (6.9%) and Villa Hermosa (5.7%), this being different with lower prevalences than those found in this study. However, the communes found to have the highest prevalence (San Javier, Belén, and Guayabal), were not sampled or had ranges lower than 2.8% in the aforementioned study. These variations in seroprevalence could relate to the diagnostic techniques used, where the methodology developed by Agudelo et al. (2012), was performed using a test based on solid phase chromatographic immunoassay with the “Antigen Rapid C Brucella Ab Test Kit”. This test can present false negatives or positives because of cross-reaction with other varieties of Brucella spp. (Sánchez et al., 2014), compared to the PARP-2ME test that was developed in this study, which detects specific antibodies for B. canis with a high sensitivity (Castrillón et al., 2019). San Javier continues to be the most prevalent commune for B. canis in relation to the preliminary study previously performed (Castrillón et al., 2019).
As previously reflected, the Buenos Aires commune, in addition to being found to be at greatest risk for canine Leptospirosis infection in this study, was determined as being the one with the highest seroprevalence for B. canis in 2012 (Agudelo et al., 2012). Castrillón et al. (2019) demonstrated that there is greater susceptibility to L. canicola and L. grippothyphosa in the communes of Villa Hermosa and Santa Cruz, a fact that could not be supported in this study. However, both communes presented one of the highest seroprevalences for this agent, with 12% and 11%, respectively (Figure 1).
The geographical location of the three communes with the largest prevalence of B. canis is to the south west of the city and to the east of the city are the two communes with the greatest finding for Leptospira spp. (see figure 1). This suggests that they could be the factors contributing to a greater circulation of said agents in these areas. This could be addressed in future research to enable the development of prevention and control measures by commune for this type of zoonosis.
Although the highest percentage of canines positive for B. canis were reported to be distributed within the age range of 13-36 months, it was not possible to find an age-related risk factor for this agent. Other studies have evidenced that dogs older than 4 years are more susceptible to Leptospira spp. than those aged one year (Meeyam et al., 2006; Ward et al., 2004). This could be related to what was reported in this study where canines 37-48 months (3-4 years) of age had 4.2 times the risk of being affected by this agent (see Table 2). This could be because adult dogs could come into greater contact with contaminated environments or animals, thus allowing for reinfection over the years and maintaining a high level of antibodies over time.
There is evidence of few studies performed on both zoonotic risk diseases, which reflects insufficient global interest in the behavior of these agents among canine populations and their relationship with the condition of people who enter into close contact with positive individuals. This further highlights the importance of prevalence studies that help understand the epidemiologic behavior of these types of agents and the development of preventive measures. Moreover, it is advisable to perform strict testing of both agents in canine populations in kennels, shelters, and foster homes, because of overcrowding and the arrival of stray animals that could be infected, thus facilitating their continuity and posing a greater transmission risk to possible adopters (Castrillón et al., 2013; Flores et al., 2017; Langoni et al., 2015; Miotto et al., 2018; Ruíz et al., 2010).
Conclusion
Canines positive for Brucellosis and Leptospirosis were found in all the sampled communes of Medellín, which shows a permanent circulation of these agents in the city and a possible contagion risk for humans who come into contact with positive canines or their contaminated dispersal areas, such as parks and water sources. Moreover, there was evidence of communes where there is a greater risk of infection of both diseases, this being a warning for the prompt application of preventive measures in the most affected communes.
Acknowledgements
We thank the Biogenesis Research Group of the University of Antioquia and to the Municipality of Medellín’ s Ministry of Health for the development and financing of this project.
References
Agudelo, P.; Castro, B.; Rojo, R. et al. Seroprevalencia y factores de riesgo para brucelosis canina en perros domésticos de once comunas de la ciudad de Medellín-Colombia. Revista de Salud Pública,v.14, n.4, p.644-656, 2012.
Álvarez, L.; Calderón, A.; Rodríguez, V. et al. Seroprevalencia de leptospirosis canina en una comunidad rural del municipio de Ciénaga de Oro, Córdoba (Colombia). Revista U.D.C.A Actualidad & Divulgación Científica,v.14, n.2, p.75-81, 2011.
Ardoino, S.M.; Baruta, D.A.; Toso, R.E. Brucelosis canina. Ciencia veterinaria,v.8, n.1, p.50-61, 2017.
Carreño, L.A.; Salas, D.; Beltrán, K.B. Prevalencia de leptospirosis en Colombia: revisión sistemática de literatura. Revista de Salud Pública,v.19, n.2, p.204-209, 2017.
Castrillón, L.; Giraldo, C.A.; Sánchez, M.M. et al. Factores asociados con la seropositividad a Brucella canis en criaderos caninos de dos regiones de Antioquia, Colombia. Cadernos de Saúde Pública,v.29, n.10, p.1975-1987, 2013.
Castrillón, L.; López, L.; Sancheze, R. et al. Prevalence of presentation of some zoonotic agents transmitted by canines and felines in Medellín, Colombia. Revista Mvz Cordoba,v.24, n.1, p.7119-7126, 2019.
Delaude, A.; Rodriguez, S.; Dreyfus, A. et al. Canine leptospirosis in Switzerland—a prospective cross-sectional study examining seroprevalence, risk factors and urinary shedding of pathogenic leptospires. Preventive veterinary medicine,v.141, p.48-60, 2017.
Echeverri, L.M.; Penagos, S.; Castañeda, L. et al. Características sociodemográficas y clínicas de pacientes con infección por Leptospira spp. atendidos en cuatro centros hospitalarios de Medellín, Colombia, 2008-2013. Biomédica,v.37, n.1, p.62-67, 2017.
Flores, B.J.; Pérezz, T.; Fuertes, H. et al. A cross-sectional epidemiological study of domestic animals related to human leptospirosis cases in Nicaragua. Acta tropica,v.170, p.79-84, 2017.
Giraldo, C.A.; Ruiz, Z.T.; Olivera, M. Brucella canis en Medellín (Colombia), un problema actual. Revista U.D.C.A Actualidad & Divulgación Científica,v.12, n.1, p.51-57, 2009.
Langoni, H.; Ponte, M.C.d.; Barbosa, D. et al. PESQUISA DE ANTICORPOS E DE DNA DE Leptospira spp. EM SORO CANINO. Veterinária e Zootecnia,v.22, n.3, p.429-436, 2015.
Lucero, N.E.; Maldonado, P.L.; Kaufman, S. et al. Brucella canis causing infection in an HIV-infected patient. Vector-Borne and Zoonotic Diseases,v.10, n.5, p.527-529, 2010.
Manias, V.; Nagel, A.; Mollerach, A. et al. Endocarditis por Brucella canis: primer caso documentado en un paciente adulto en Argentina. Revista argentina de microbiología,v.45, n.1, p.50-53, 2013.
Meeyam, T.; Tablerk, P.; Petchanok, B. et al. Seroprevalence and risk factors associated with leptospirosis in dogs. Southeast Asian journal of tropical medicine and public health,v.37, n.1, p.148, 2006.
Miotto, B.A.; Guilloux, A.G.A.; Tozzi, B.F. et al. Prospective study of canine leptospirosis in shelter and stray dog populations: Identification of chronic carriers and different Leptospira species infecting dogs. PloS one,v.13, n.7, 2018.
Olivera, M.; Di-Lorenzo, C. Aislamiento de Brucella canis en un humano conviviente con caninos infectados. Informe de un caso. Colombia Médica,v.40, n.2, p.218-220, 2009.
Olivera, M.; Giraldo, C.; Di-Lorenzo, C. Identificación por PCR de Brucella canis en sangre y leche canina: Reporte de un caso. Archivos de medicina veterinaria,v.43, n.3, p.295-298, 2011.
Pulido, A.; Carreño, G.; Mercado, M. et al. Situación epidemiológica de la leptospirosis humana en Centroamérica, Suramérica y el Caribe. Universitas Scientiarum,v.19, n.3, p.247-264, 2014.
Ruíz, J.D.; Giraldo, C.A.; López, L.V. et al. Brucella canis seroprevalence in stray dogs from" Centro de Bienestar Animal" La Perla", Medellín (Colombia), 2008. Revista Colombiana de Ciencias Pecuarias,v.23, n.2, p.166-172, 2010.
Salas. INFORME DE EVENTO LEPTOSPIROSIS, COLOMBIA, 2017. Equipo Funcional Enfermedades Trasmitidas por Vectores. Colombia: INS y MiniSalud, 2018.
Sánchez, M.; Giraldo, C.A.; Olivera, M. Infección por Brucella canis en humanos: propuesta de un modelo teórico de infección a través de la ruta oral. Infectio,v.17, n.4, p.193-200, 2013.
Sánchez, M.; Ortiz, L.F.; Castrillón, L.L. et al. Application of a polymerase chain reaction test for the detection of Brucella canis from clinical samples of canines and humans. Revista Colombiana de Ciencias Pecuarias,v.27, n.1, p.3-11, 2014.
Souza, T.D.; Carvalho, T.F.; Silva, J.P. et al. Tissue distribution and cell tropism of Brucella canis in naturally infected canine foetuses and neonates. Scientific reports,v.8, n.1, p.1-10, 2018.
Ward, M.P.; Guptill, L.F.; Wu, C.C. Evaluation of environmental risk factors for leptospirosis in dogs: 36 cases (1997–2002). Journal of the American Veterinary Medical Association,v.225, n.1, p.72-77, 2004.
* The project was financed by the Municipality of Medellin’s Department of Health through contracts 4600064258 and 4600070399.
Como citar: López-Diez L., Ortiz-Román L., Sanchez-Nodarse R., Sanabria-Gonzalez W., Henao-Correa E., Olivera-Angel M. Seroprevalence of Brucella canis and Leptospira spp. in canines in the city of Medellín, Colombia. Revista Veterinaria y Zootecnia. n, v. 14, n. 1, p. 00-00, 2020. http://vetzootec.ucaldas.edu.co/index.php/component/content/article?id=285. DOI: 10.17151/vetzo.2020.14.1.4
Esta obra está bajo una Licencia de Creative Commons Reconocimiento CC BY
|