|
Uterine serosal inclusion cysts in a nulliparous bitch: A case report
Daniela Cortés-Beltrán1
1 Department of Animal Health, Universidad Nacional de Colombia, School of Veterinary Medicine and Zootechnics, Animal Reproduction Clinic, Reproductive Biotechnology Laboratory, Research Group on Animal Reproduction and Health of Hato, Bogotá DC–Colombia.
This email address is being protected from spambots. You need JavaScript enabled to view it.
Recibido: 2 agosto de 2019, Aprobado: 30 septiembre de 2019, actualizado: 18 diciembre de 2019
DOI: 10.17151/vetzo.2020.14.1.5
ABSTRACT. Introduction: Uterine serosal inclusion cysts are structures derived from mesothelial cells attached to the uterus’ antimesometrial side and are mainly present in females during the postpartum period. They are physiologically inactive and do not interfere with reproductive functioning of the affected animals. It is typically an incidental finding in laparotomy and can be considered as a differential diagnosis when there is evidence of uterine content during abdominal sonography. Although it is not regarded as a pathology that affects animal welfare, its primary therapy is ovariohysterectomy. Case report: A 12-year-old mixed-breed bitch was presented at the Animal Reproduction Clinic of the Universidad Nacional de Colombia. The reason for consultation was abdominal distension, vulvar discharge, polydipsia, polyuria, and excessive vulvar licking. The diagnosis included clinical findings compatible with estrus, vaginal cytology indicative of estrus, uterine contents observed by sonography, and high serum progesterone levels. Hence, ovariohysterectomy therapy was recommended and performed. After surgery, a small amount of dark content was present in the uterine cavity along with anovulatory follicles, and as an incidental finding, several uterine serosal inclusion cysts were detected during the procedure. Conclusion: The presentation of this pathology is rare in nulliparous bitches, but it is of vital importance to consider it as a differential diagnosis in patients with signs of estrus and findings compatible with uterine content on sonography.
Keywords: uterine content, ovarian cyst, uterine cyst, sonography
Quistes de inclusión de la serosa uterina en una perra nulípara: Reporte de caso
RESUMEN. Introduccion: Los quistes de inclusión de la serosa uterina son estructuras derivadas de células mesoteliales adheridas sobre el lado antimesometrial del útero y que se presentan principalmente en hembras postparto. Son fisiológicamente inactivos y no interfieren en la función reproductiva de los animales. Usualmente es un hallazgo incidental a la laparotomía y puede ser considerado como un diagnóstico diferencial cuando hay evidencia de contenido uterino a la ultrasonografía abdominal. El principal plan terapeutico es la ovariohisterectomía aunque no se considera una patología que afecte el bienestar del animal. Reporte de caso: Asiste a la Clínica de Reproducción Animal de la Universidad Nacional un hembra canina de 12 años de edad, mestiza cuyo motivo de consulta fue distensión abdominal, secreción vulvar, polidipsia, poliuria y lamido vulvar excesivo. Se diagnosticó hallazgos clínicos compatibles con estro, citología vaginal compatible con estro, contenido uterino por ultrasonografía y niveles elevados de progesterona sérica; se instauró como plan terapéutico ovariohisterectomía. Después de la cirugía, el útero tenía contenido oscuro en poca cantidad, se encontraron folículos anovulatorios y como hallazgo incidental durante el procedimiento se encontraron varios quistes de inclusión de la serosa uterina. Conclusión: La presentación de esta patología es poco frecuente en hembras nulíparas, pero queda demostrado que es de vital importancia listarla como diagnóstico diferencial ante pacientes con hallazgo compatibles con contenido uterino a la ultrasonografía y signología de celo.
Palabras clave: contenido uterino, quistes ováricos, quistes uterinos, ultrasonografía
Introduction
Cystic endometrial hyperplasia, pseudo‐placentational endometrial hyperplasia, uterine polyps, adenomyosis, subinvolution of placental sites, and serosal inclusion cysts are included among the uterine diseases involving endometrial and mesometrial growth abnormalities (Schlafer & Gifford, 2008).
Uterine serosal inclusion cysts are thin-walled structures present on the serosal surface of the uterus, often forming small grape-like clusters (Saxena et al., 2006). These structures are derived from mesothelial cells (Sevimli et al., 2012), develop along linear folds on the outer uterine surface, and adhere to the mesothelial serosal covering (Sevimli et al., 2012; Sathiamoorthy & Raja, 2012). They are a result of rapid growth of the uterus and contractions of the uterus that occur during uterine involution during the postpartum period (Sevimli et al., 2012). During postpartum uterine involution, these structures become trapped due to rapid contraction of the myometrium (Sathiamoorthy et al., 2014). They are usually an incidental finding observed during exploratory laparotomy (Arnold et al., 1996).
This case report describes the clinical signs and macroscopic features of uterine serosal inclusion cysts in a nulliparous bitch.
Case report
A 12-year-old, golden, mixed-breed, reproductively sound, nulliparous bitch with up-to-date deworming and vaccination was presented at the Animal Reproduction Clinic of Universidad Nacional de Colombia. Its medical record included a history of pseudopregnancy and mastitis. The owners reported abdominal distension, vulvar discharge in the form of drops, excessive vulvar licking, polydipsia, polyuria, frequent bowel movements (up to 4 times a day), occasional tenesmus, and vulvar edema for 5 days. The owners also reported that the last heat occurred more than a year ago, the duration and commencement of which were unknown.
Clinical examination revealed an alert, docile, and clinically stable patient with a body condition 3/5, weight 10.23 kg, pink mucous membranes, 2-second capillary refill, heart rate 110 bpm, respiratory rate 21 rpm, and temperature 38.7°C. As an abnormal finding, mild abdominal distension was evident, without the presence of specific abnormal structures on abdominal palpation. Upon examination of the reproductive organs, tail flagging, positive vaginal reflex, moderate vulvar edema, and non-developed mammary gland without secretion could be observed. The list of clinical findings proposed included mild abdominal distension, historical vaginal discharge, excessive vulvar licking, polydipsia, polyuria, frequent bowel movements with occasional tenesmus, and vulvar edema. The following differential diagnoses were considered: cystic endometrial hyperplasia-pyometra complex, urinary tract infection, estrus, and vaginitis.
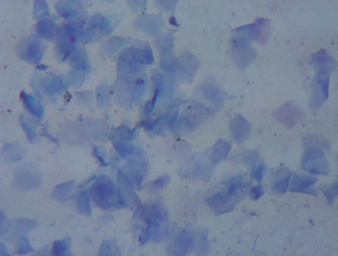
Diagnosis was performed by vaginal cytology using Wright’s stain, which was subsequently observed under a conventional light microscope (40× magnification), revealing a dirty background and high cellularity with a predominance of superficial cells, of which 90% and 10% were keratinized and non-keratinized, respectively, suggestive of estrus. No inflammatory changes were observed. (Figure 1).
Figure 1. Vaginal cytology using Wright’s stain (400× magnification). High cellularity with predominance of superficial cells, of which 90% and 10% were keratinized and non-keratinized, respectively.
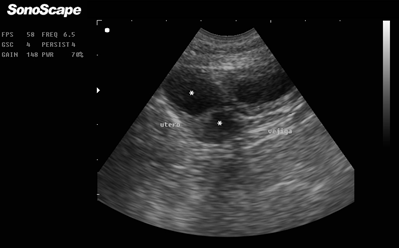
Additionally, sonography was performed (SonoScape AV5, mode B, 6.5 MHz Micro Convex transducer), which revealed multiple non-echogenic cavities cranial to the urinary bladder, which were compatible with uterine contents (Figure 2).
Figure 2. Abdominal B-mode sonography using Micro Convex probe and 6.5 MHz. Urinary bladder (right side) and two non-echogenic areas compatible with uterine content (asterisks on the left side).
Based on the conducted diagnostic procedures, it was possible to determine that the vaginal cytology results were compatible with estrus and that the abdominal sonography findings suggested uterine content. Based on these findings, uterine content was added to the list of symptoms and polycystic ovaries to the differential diagnoses. Ovariohysterectomy was recommended, along with a new control visit for follow-up and paraclinical examination. During the 3 days after consultation, the owners observed an increase in abdominal distension, in addition to persistent excessive vulvar licking, polydipsia, –polyuria, and occasional tenesmus. General clinical examination did not reveal any additional abnormalities, although reproductive organ examination showed absence of tail flagging. A new vaginal cytology was performed, which revealed a dirty background and high cellularity with a predominance of keratinized (80%) and non-keratinized (20%) superficial cells. Vaginoscopy revealed pale and rough vaginal mucosa. Additionally, there was no evidence of abnormal structural findings. A new sonography was performed wherein it was still possible to identify multiple non-echogenic cavities cranial to the bladder, compatible with uterine contents. Blood samples were collected to perform blood counts, which were within the normal range.
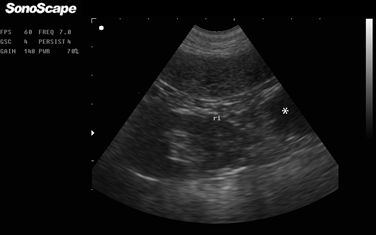
On the 10th day of progress, the patient was taken for a follow-up visit where the owners reported deterioration of the patient’s state, with presentation of hyporexia accompanying polydipsia and marked polyuria. Similarly, they noticed an increase in abdominal distension and persistent excessive vulvar licking. Clinical examination revealed physiological constants within normal ranges and an enlargement of the submandibular lymph nodes and the popliteal lymph nodes of the right hind limb. The abdominal distension remained mild. A new vaginal cytology and vaginoscopy were performed, which revealed a pattern similar to the findings of the previous tests. Additionally, digital examination was performed, which showed no evidence of masses at the vaginal vestibule level. Abdominal sonography determined the presence of multiple cavitary and non-echogenic structures, measuring approximately 21.2 mm in diameter and 42.4 mm in length, toward the caudal pole of the left kidney (Figure 3), compatible with uterine content and anovulatory follicles. Lastly, a blood sample was taken for measuring progesterone levels, which were 52 ng/ml.
Figure 3. Abdominal B-mode sonography using Micro Convex probe and 6.5 MHz. Left kidney and evidence of a non-echogenic area caudal to it (asterisk on the left side) compatible with anovulatory follicles.
Treatment
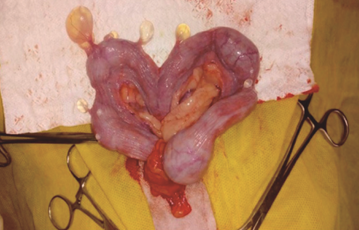
An ovariohysterectomy was performed after a 12 hour-fasting and under inhaled anesthesia. Subsequently, after antiseptic treatment of the medial abdomen, a laparotomy was performed, which exposed both uterine horns and revealed the presence of multiple cystic structures, ranging between 0.4 and 0.8 cm, attached to the uterine serosa with translucent yellowish fluid in its interior (Figure 4).
Figure 4. Multiple cystic structures on the uterus observed during laparotomy.
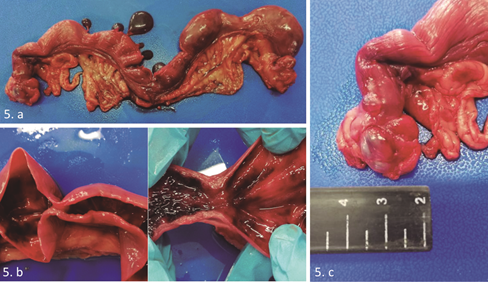
After extraction of the reproductive tract, a macroscopic evaluation was performed, confirming the presence of multiple cysts attached to the uterine serosa, which was consistent with uterine serosal inclusion cysts (Figure 5.a). Considering this, a longitudinal cut was made on both uterine horns, revealing bloody discharge and septa over the endometrium (Figure 5.b). Upon evaluation of the ovaries, the left ovary showed multiple bulging structures containing serous fluid, which was consistent with follicular cysts (Figure 5.c), whereas the right ovary showed presence of corpora lutea. The uterine content was then analyzed by cytology, revealing countless red blood cells and absence of polymorphonuclear leukocytes.
Figure 5. Macroscopic evaluation of the reproductive tract. 5.a. Presence of uterine serosal inclusion cysts. 4.c Ovarian bursa. Polycystic right ovary.
Lastly, the recovery and clinical evolution of the patient was favorable after treatment, with complete resolution of the symptoms presented at the time of consultation at the Animal Reproduction Clinic.
Discussion
Uterine serosal inclusion cysts are thin-walled cystic structures containing non-viscous fluid, which are located on the serous surface of the uterus (Schlafer & Gifford, 2008). The cysts are generally multiple, united by a thin stem of variable size of the serous membrane, and are located on the antimesometrial side of the uterus (Sathiamoorthy & Raja, 2012; McEntee, 1990). These structures are predominantly found in older multiparous females, and their location is generally focal (Arnold et al., 1996; Schlafer & Gifford, 2008). There are reports on cases of multifocal distribution, which are distributed into groups on the peritoneal covering of the horns and uterine body (Schlafer & Gifford, 2008; Sevimli et al., 2012). These structures are typically observed in postpartum females during uterine involution (Maggie et al., 2015), where the rapid contraction of the myometrium produces folds in portions of the mesothelium, which ultimately become uterine serosal inclusion cysts (McEntee, 1990; Sathiamoorthy & Raja, 2012). Their presentation can also be related to laparotomies or C-section surgery wherein the uterus has been extensively manipulated (Schlafer & Gifford, 2008). In the present case, the patient had no history of deliveries and a mammary gland evaluation did not reveal development; therefore, these structures were considered to have a different origin than that reported in the literature. The cystic structures found were randomly distributed over both uterine horns, mainly on the right horn. As stated in the literature, it is possible that the inclusion cysts observed in the patient had developed from the peritoneum covering the surface of the uterus, starting as mild peritoneal reactivity areas and progressing into trapping of small serosa pieces (Schlafer & Gifford, 2008).
These cysts are considered to be clinically benign and physiologically inactive, with no ability to interfere with the reproductive function (McEntee, 1990); however, the presentation of these cysts may be associated with hormonal dysfunction and, occasionally, pyometry (Schlafer & Miller, 2007). In this case, another cause for the origin of these structures may be hormonal dysfunction, because, according to the findings in vaginal cytology, estrogenemia was indicated, which may be related to the finding of polycystic ovaries, possibly due to a partial failure of ovulation. According to Knauf et al. (2014), estrogens increase uterine and myometrium contractions, generating folds in different areas of the mesothelium and consequently generating uterine serosal inclusion cysts, a phenomenon that has been reported in female buffaloes (Saxena et al., 2006; Sevimli et al., 2012).
Based on the clinical findings, cystic endometrial hyperplasia-pyometra complex was proposed as a differential diagnosis, as the patient presented mild abdominal distension due to the presence of uterine content and a history of vulvar discharge. Additionally, sonograms of the abdominal cavity were suggestive of uterine content, a common finding in animals presenting this pathology (Sathiamoorthy & Raja, 2012). Urinary tract infection and vaginitis were included in the list of differential diagnoses given the history of vulvar discharge and constant vulvar licking, commonly caused by an ascending bacterial infection of the normal vaginal microbiota (Soderberg, 1986). Finally, polycystic ovaries were added to the list of differential diagnoses, since bitches with this pathology secrete high amounts of estrogens, causing irregularities of the estrus (Maggie et al., 2015). This would explain the behavioral, physical, and cytological changes observed in the patient, such as tail flagging, positive vaginal reflex, vulvar edema, vaginal cytology with high cellularity and a predominant keratinized superficial cells, and vaginoscopy with pale and rough vaginal mucosa.
Conversely, the influence of progesterone secreted from the corpora lutea found in the right ovary, which is a predisposing factor for hyperplasia of the endometrium and endometrial glands, decreases myometrial contractions and inhibits the local response of leukocytes to infection, allowing bacterial proliferation within the uterine lumen (Schlafer & Gifford, 2008; Maggie et al., 2015). In this case, it was observed that a heat had not occurred for more than a year, as indicated by the owners, because corpora lutea were found during the macroscopic evaluation of the reproductive tract, in addition to high levels of progesterone (52 ng/ml).
Ovariohysterectomy we performed where, when exposing both uterine horns, it was possible to identify multiple cystic structures with features reported in the literature, such as translucent yellowish non-viscous liquid within thin walls forming small grape-like clusters of varying sizes (Schlafer, 2012; Schlafer & Gifford, 2008). This finding is generally observed during ovariohysterectomy in bitches (Arnold et al., 1996; Kennedy & Miller, 1993; Schlafer & Gifford, 2008; Vural et al., 2004), female cats (Godfrey & Silkstone, 1998), and female buffaloes in processing plants (Saxena et al., 2006; Sevimli et al., 2012), all of which are incidental findings. The macroscopic inspection revealed the presence of abundant blood content inside both uterine horns like sacculations, as reported in a German shepherd bitch with uterine serosal inclusion cysts with abnormal vaginal discharge for several weeks. The authors report that uterine serosal inclusion cysts are not always physiologically inactive and may sometimes be associated with the development of pyometra or some other uterine condition (Arnold et al., 1996; Sathiamoorthy et al., 2014).
The analysis of uterine content by cytology evidenced countless red blood cells, similar to that reported in a case report of a 4-year old bullmastiff bitch. In accordance with the cases reported, the authors evidenced this same finding, which was related to conditions associated with follicular functional cysts, such as iatrogenic hyperestrogenism or hyperestrogenism (Sánchez, 2015). Likewise, other authors have reported that endocrine activity of ovarian cysts can be a predisposing factor for cystic endometrial hyperplasia-pyometra in bitches, because of a reported correlation between the cysts and the presentation of this uterine pathology, as they usually cause hyperestrogenism, which is associated with prolonged progestagenic stimulation predisposed to cystic endometrial hyperplasia-pyometra (Knauf et al., 2014; Sánchez, 2015).
The prevalence of this disease is still unclear; however, it appears to be low, given that some studies on reproductive pathologies of stray bitches report a 5% prevalence of uterine serosal inclusion cysts in the studied females (Ortega-Pacheco et al., 2006).
Conclusion
The occurrence of uterine serosal inclusion cysts in a nulliparous bitch is a very unusual phenomenon. However, considering the clinical findings of this report, it can be concluded that high levels of estrogens, which also caused changes in vaginal cytology and patient behavior, could be an important factor for the occurrence of uterine serosal inclusion cysts without delivery. Hence, it is recommended to consider this pathology as a differential diagnosis, in the presence of sonography findings consistent with cystic endometrial hyperplasia-pyometra complex and persistent ovarian follicles.
References
Arnold, S. et al. Uterine serosal inclusion cysts in a bitch. J Small Anim Pract, v. 37, p. 235-237, 1996.
Godfrey, D. L; Silkstone, M. A. Uterine serosal inclusion cysts in a cat. Vet. Res, v. 142, p. 673, 1998.
Knauf, Y. et al. Gross Pathology and Endocrinology of Ovarian Cysts in Bitches. Reproduction in Domestic Animals, v. 49, n. 3, p. 463–468, 2014.
Kennedy, P. C; Miller, R. The female genital system. In: Jubb, K. V. F; Kennedy, P. C; Palmer, A. C. (Ed). Pathology of Domestic Animals. 4th Ed. London: Academic Press, 1993. p. 349.
Maggie, F. T. et al. Pathological Study on Female Reproductive Affections in Dogs and Cats at Alexandria Province, Egypt. Alexandria Journal of Veterinary Sciences, v. 46, p. 74-82, 2015.
McEntee K. The Uterus: Degenerative and inflammatory lesions. In: McEntee, K. (Ed). Reproductive Pathology of Domestic Animals. San Diego: Academic press, 1990, p. 158.
Ortega-Pacheco, A. et al. Reproductive patterns and reporductive pathologies of spray bitches in the tropics. Theriogenology, v.67, p.382-390, 2006.
Sathiamoorthy, T; Raja, S. Uterine Serosal Inclusion Cysts in a Bitch. Indian Vet. J, v. 89, n. 12, p. 89-90, 2012.
Sathiamoorthy, T. et al. Uterine serosal inclusion cysts coupled with pyometra in a bitch. Indian Vet. J, v. 35, n. 1, p. 61-62, 2014.
Sánchez, R. A. Hematometra e Hiperplasia Endometrial Quística en una Perra: Descripción de un Caso. Revista de Investigaciones Veterinarias Del Perú, v. 26, n. 1, p. 146, 2015.
Saxena, G. et al. Pathological conditions in genital tract of female buffaloes (Bubalus bubalis). Pakistan Vet J, v. 26, p. 91-93, 2006.
Sevimli, A; Ozenc, E; Acar, D. B. Oviduct cyst observed together with a uterine serosal inclusion cyst in the Anatolian water buffalo–a case report. Acta Veterinaria Brno, v. 81, n. 3, p. 235–237, 2012.
Schlafer, D. H. Diseases of the Canine Uterus. Reprod Dom Anim, v. 47, n. 6, p. 318-322, 2012.
Schlafer, D. H; Gifford, A. T. Cystic endometrial hyperplasia, pseudo-placentational endometrial hyperplasia, and other cystic conditions of the canine and feline uterus. Theriogenology, v. 70, n. 3, p. 349-358, 2008.
Schlafer, D. H; Miller, R. B. Female genital system. In: Jubb, K. V. F; Kennedy, P. C; Palmer, A. C. (Ed). Pathology of Domestic Animals, 4th Ed. London: Academic Press, 2007, p. 429–564.
Soderberg, S. F. Vaginal Disorders. Veterinary Clinics of North America: Small Animal Practice, v. 16, n. 3, p. 543–559, 1986.
Vural, S. A; Haligur, M; Ozenc, E. Uterine serosal inclusion cysts in dogs: Pathomorphological and immunohistochemical findings (in German). Kleintierpraxis, v. 49, p. 375-377, 2004.
Como citar: Cortés-Beltrán D., Pinzón-Osorio1 C.A., Lozano-Márquez H., Zambrano-Varón J.L., Ruíz-Cristancho A., Jiménez-Escobar C. Uterine serosal inclusion cysts in a nulliparous bitch: A case report. Revista Veterinaria y Zootecnia. n, v. 14, n. 1, p. 00-00, 2020. http://vetzootec.ucaldas.edu.co/index.php/component/content/article?id=286. DOI: 10.17151/vetzo.2020.14.1.5
Esta obra está bajo una Licencia de Creative Commons Reconocimiento CC BY
|